eISSN 2444-7986
DOI: https://doi.org/10.14201/orl.33191
REVIEW ARTICLE
PARANEOPLASTIC NEUROLOGIC SYNDROMES ASSOCIATED WITH NASOPHARYNGEAL CARCINOMA: A SYSTEMATIC REVIEW
Síndromes neurológicos paraneoplásicos asociados al carcinoma nasofaríngeo: una revisión sistemática
Javier RIANCHO  1, 2, 3, 4; Eloy RODRIGUEZ-RODRIGUEZ
1, 2, 3, 4; Eloy RODRIGUEZ-RODRIGUEZ  2, 4, 5; Andrea MARTINEZ-CAMERANO
2, 4, 5; Andrea MARTINEZ-CAMERANO  6; Julia FERNANDEZ-ENSEÑAT
6; Julia FERNANDEZ-ENSEÑAT  6; Carmelo MORALES-ANGULO
6; Carmelo MORALES-ANGULO  2, 4, 6
2, 4, 6
1 Department of Neurology Hospital General Sierrallana, Torrelavega, Spain
2 Institute of Research Valdecilla (IDIVAL), Santander, Spain
3 CIBERNED, Madrid, Spain
4 Faculty of Medicine, University of Cantabria, Santander, Spain
5 Department of Neurology. University Hospital “Marqués de Valdecilla” Santander, Spain
6 Department of Ear Nose and Throat. University Hospital “Marqués de Valdecilla” Santander, Spain
Correspondence: Carmelo.morales@unican.es
Reception date: April 27, 2025
Date of Acceptance: June 8, 2025
Publication date: June 14, 2025
Date of publication of the issue: To be published
Conflict of interest: The authors declare no conflicts of interest
Rights policy and self-archive: the self-archive of the post-print version (SHERPA/RoMEO) is allowed
License CC BY-NC-ND. Creative Commons Attribution-Noncommercial-NoDerivate Works 4.0 International
University of Salamanca. Its commercialization is subject to the permission of the publisher
SUMMARY: Introduction and objectives: Paraneoplastic syndromes (PS) are a heterogeneous group of cancer-associated disorders caused by mechanisms other than metastases, metabolic, nutritional deficits, infections, or side effects of cancer treatment PS appear in 1-7.4 % of all cancer patients. Paraneoplastic neurological syndromes (PNS) associated with nasopharyngeal carcinoma (NC) are very rare. The objective of this review is to characterize the PNS associated with NC. Methods: A systematic review of cases of PNS associated to NC was carried out. The following databases were used for the search: Pubmed, Web of Science and Cochrane library. As search terms we used the keywords: “paraneoplastic syndrome” and “nasopharyngeal carcinoma”. Results: We included a total of 11 patients in the review. The PNS most frequently identified were cerebellar degeneration, limbic encephalitis, and opsoclonus-myoclonus syndrome. Auto antibodies against neural structures were identified in 5 cases (anti-Yo, anti-Hu and anti-RI). Regarding neurological prognosis, neurological symptoms did not improve or improved only partially after both primary tumor and steroids and immunoglobulins. Conclusions: Clinicians should consider the possibility of a paraneoplastic etiology when caring for patients with NC who present with subacute neurological symptoms. The prognosis of PNS after treatment is poor.
KEYWORDS: paraneoplastic; myelitis; nasopharyngeal carcinoma; antineural antibodies; head and neck carcinoma
RESUMEN: Introducción y objetivos: Los síndromes paraneoplásicos (SP) son un grupo heterogéneo de trastornos asociados al cáncer, causados por mecanismos distintos a las metástasis, déficits metabólicos o nutricionales, infecciones o efectos secundarios del tratamiento oncológico. Los SP aparecen en el 1–7.4 % de todos los pacientes con cáncer. Los síndromes neurológicos paraneoplásicos (SNP) asociados al carcinoma nasofaríngeo (CN) son muy raros. El objetivo de esta revisión es caracterizar los SNP asociados al CN. Métodos: Se realizó una revisión sistemática de los casos publicados de SNP asociados al CN. Se utilizaron las siguientes bases de datos para la búsqueda: Pubmed, Web of Science y Cochrane Library. Como términos de búsqueda, se emplearon las palabras clave: "síndrome paraneoplásico" y "carcinoma nasofaríngeo". Resultados: Se incluyeron un total de 11 pacientes en la revisión. Los SNP más frecuentemente identificados fueron la degeneración cerebelosa, la encefalitis límbica y el síndrome opsoclono-mioclono. Se identificaron autoanticuerpos contra estructuras neuronales en 5 casos (anti-Yo, anti-Hu y anti-RI). En cuanto al pronóstico neurológico, los síntomas neurológicos o bien no mejoraron o lo hicieron parcialmente tras el tratamiento tanto del tumor primario como mediante corticoides e inmunoglobulinas. Conclusiones: Se debe considerar la posibilidad de una etiología paraneoplásica al tratar a pacientes con CN que presenten síntomas neurológicos subagudos. El pronóstico de los SNP tras el tratamiento es malo.
PALABRAS CLAVE: paraneoplásico; mielitis; carcinoma nasofaríngeo; anticuerpos antineuronales; carcinoma de cabeza y cuello
INTRODUCTION
Paraneoplastic syndromes (PS) are a heterogeneous group of cancer-associated disorders caused by mechanisms other than metastases, metabolic, nutritional deficits, infections, or side effects of cancer treatment [1]. PS appear in 1-7.4 % of all cancer patients and they may precede, follow or be concurrent with the clinical manifestation of a malignant neoplasm [2].
Nasopharyngeal carcinoma (NC) is uncommon in Western countries but is considered endemic in the South-east parts of China [3, 4]. The risk factors associated to NC are tobacco, hereditary trends, race, and in cases of undifferentiated NC, Epstein-Barr virus (EBV) infection [5]. The most frequent presentation symptoms of NC are cervical lymphadenopathy, middle ear and nasal problems. However, a small group of patients may develop clinical manifestations related to PS [5].
It is difficult to know the exact incidence of PS associated to NC because numerous cases with clinical manifestations related to the invasion of tumour-adjacent structures or to distant metastases have been published as false-PS [6]. The constellation of PS associated to NC is quite extent including connective tissue diseases, particularly dermatomyositis and scleroderma, paraneoplastic endocrine syndromes as the syndrome of inappropriate secretion of antidiuretic hormone (SIADH), paraneoplastic hematologic syndromes as tumor fever, leukemoid reaction and immune thrombocytopenia, paraneoplastic osteoarticular syndromes as finger clubbing and, more rarely, paraneoplastic neurologic syndromes (PNS) [2, 5, 7]. PNS are most associated to small cell lung cancer (SCLC) but their association with other solid neoplasms, including a few cases related with NC, have been reported [2].
The objective of our study was to carry out a systematic review of the literature of published cases of PNS associated with NC with the aim of better characterised this infrequent condition.
MATERIAL AND METHODS
For the literature review, we performed a systematic review of the previously published cases of PNS associated to NC until March 31, 2025. Pubmed, Web of Science and Cochrane Library were used for the cases identification. As search terms, we used “nasopharynx carcinoma” and “paraneoplastic syndrome”. The review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines with modifications adapted to the type of study [8]. Regardless the language, all published articles were analysed. Data extraction was carried out by two of the authors according to previously established criteria. All abstracts and research titles that resulted from the initial search were analyzed. After the initial selection, the full text of the selected articles was investigated (Figure 1).
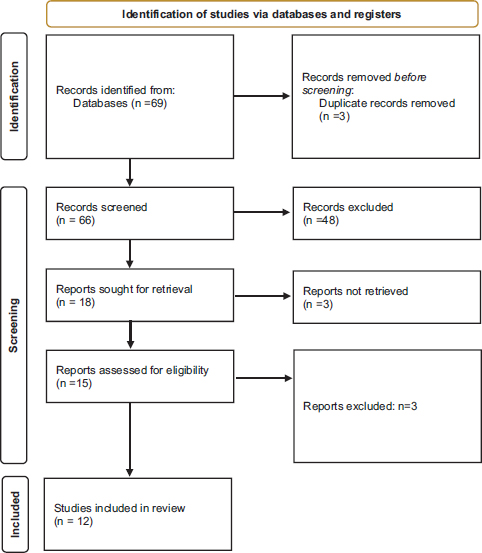
Figure 1. PRISMA diagram with the selection of articles
We only included those patients with the confirmation of a PNS following the criteria previously established by Grau et al [9]. In this regard, we exclude other neurological conditions coinciding with the carcinoma, complications related to the tumour or its treatment or any other condition that could be explained by an associated disease.
The data were included in a SPSS database, carrying out a descriptive study of the included variables.
RESULTS
The bibliographic search yielded a total of 69 results, of which 11 articles were finally detected for the analysis. Of note, all of them were case reports only including single cases. All previously published articles have been summarized in Table 1. We included a total of 12 patients in this review with a median age of 50.5 (range 39-76 years). Regarding gender, the vast majority of cases occurred in males.
Table 1. Patients with paraneoplastic syndrome associated to nasopharyngeal carcinoma included in the review
Nº/Author/Year |
Age/Sex |
Histologic subtype |
Type of PNS |
Diagnostic method |
Was the PNS the first symptom?" |
Time prior or posterior to the diagnosis of NPC |
Improvement after treatment of the primary tumor |
Phamtunchinda/1990 [10] |
51/Male |
Non-keratinizing SCC |
Sensorymotor Neuropathy |
Un |
No |
5 months after |
No improvement |
Chan/2007 [11] |
39/Male |
Un |
Simultaneous paraneoplastic predominantly motor polyneuropaty/polyradiculoneuropathy and inflammatory myopathy |
Un |
No |
Un |
Un |
Taib/2015 [12] |
50/ Female |
NPC Un |
Opsoclonus-myoclonus syndrome |
Un |
Yes |
Un |
Partial improvement |
Yu/2016 [13] |
63/ Male |
NPC Un |
Limbic Encephalitis |
AntiHu antibodies |
Yes |
Un |
NA |
Ng/2017 [14] |
76/ Male |
Undifferentiated carcinoma |
Paraneoplastic Neurological Disorder (Undefined) |
Un |
No |
Un |
Partial improvement after RT |
Bhardwaj/2019 [15] |
46/Male |
NPC Un |
Paraneoplastic cerebellar degeneration |
Anti-Yo antibodis |
Yes |
4 months |
No improvemnt with CR and corticosteroids |
Ozozen Ayas/2021 [16] |
44/Male |
NPC Un |
Cerebellar degeneration |
Un |
Un |
Un |
Un |
Tosunoglu/2022 [17] |
52/Male |
Undifferentiated carcinoma |
Limbic encephalitis |
Un |
Yes |
2 months |
Un |
Stewart/2022 [18] |
>50/ Male |
Undifferentiated carcinoma |
Opsoclonus/myoclonus syndrome |
Anti-Ri antiboides |
Yes |
1 month |
No Improvement |
Riancho/2025 [19] |
70/Male |
Non-keratinizing SCC |
Paraneoplastic myelitis |
PET-CT (anti-Yo antibodies) |
Yes |
Coincident |
Mild improvement |
Hanağası/2025 [20] |
50/Female |
Undifferentiated carcinoma |
Cervical dystonia, laryngospasm, and spastic quadriparesis |
Anti-Ri antiboides |
No |
6 months |
Partial improvement |
PNS: Paraneoplastic neurological syndrome
SCC: Squamous cell carcinoma
NPC: Nasopharyngeal carcinoma
Un: unspecified
The clinical presentation was also very heterogeneous. Cerebellar degeneration, limbic encephalitis and opsoclonus-myoclonus syndrome identified in 2 cases each, were the most frequent clinical syndromes. Antineuronal antibodies were detected in five patients: anti-Yo in two cases, anti-Ri in two cases, and anti-Hu in one case.
Undifferentiated carcinoma (four cases) and non-keratinizing squamous cell carcinoma (two cases) were the subtypes associated with the SNP. In the rest, the type of CN was not described.
In 6 of the 11 cases in which it was described, PNS symptoms appeared before the diagnosis of NC. The tumor was detected during the initial diagnostic process up to 6 months after PNS.
Regardless the satisfactory treatment of the underlying NC, the vast majority of patients did not present a significant recovery of the neurological manifestations.
DISCUSSION
The few cases included in this systematic review demonstrate how rare the appearance of PNS associated with NC is. On the other hand, there is no type of SNP especially associated with NC, since none has been described in more than 2 patients.
The pathogenesis of PNS has not been fully elucidated, but immunologic factors are thought to play an important role in many cases. A commonly accepted hypothesis suggests that the ectopic expression of neural antigens by the tumor triggers an immune response that finally targets the neurons expressing the shared antigen with the tumor [4, 19].
A variety of autoantibodies have been linked to paraneoplastic neurologic syndromes (PNS) due to the co-expression of onconeural proteins by tumors. Among these, anti-Yo antibodies are among the most prevalent and have been shown to potentially destroy Purkinje cells in the cerebellum [21]. These antibodies are characteristically associated with paraneoplastic cerebellar degeneration, although other PNS have also been linked to them. While anti-Yo antibodies are typically found in women with gynecological tumors, isolated cases have been reported in other malignancies, including prostate cancer, lung cancer, melanoma, and pleural mesothelioma [22-24]. In our review, we identified two cases associate to anti-Yo antibodies: one associated with paraneoplastic cerebellar degeneration and the other with myelitis [19].
Flanagan et al reported a series of patients with paraneoplastic myelitis [25]. As in the two cases in this review, most patients had a subacute onset with mild pleocytosis and raised proteins in CSF. In that series amphiphysin-immunoglobulin G was the most common autoantibody, appearing in almost 30 percent of patients. AntiYo antibodies were only implicated in one case [25]. The presence of anti-Yo antibodies represents a poor prognosis [25].
Anti-Ri antibodies have been described in the context of brainstem encephalitis, cerebellar syndromes, myelopathies and opsoclonus-myoclonus syndrome generally associated with SCLC and malignant gynecological tumors [26, 27]. In our review, they were found in a patient with opsoclonus-myoclonus syndrome (OMS) [18], which is characterized by rapid, chaotic and conjugated eye movements and generalized jerks with predominance in the proximal muscles [26], and in another patient with cervical dystonia, laryngospasm and spastic quadriparesis [20].Although the etiology can also be infectious or metabolic/toxic, the most common cause detected is SCLC, with anti-Ri antibodies being the most common associated antibodies [28].
Anti-Hu antibodies are most commonly associated with SCLC [29]. In our review they were found to be associated with limbic encephalitis [13].
Limbic encephalitis is an autoimmune and/or paraneoplastic disease characterized by inflammation of the limbic system and other parts of the brain. The main manifestation of limbic encephalitis is anterograde amnesia but other psychiatric and non-psychiatric symptoms such as endocrine dysfunction, sleep disturbances, apraxia and aphasia may be present [13, 17]. Limbic encephalitis is more frequently associated with SCLC [30, 31].
Paraneoplastic cerebellar degeneration is the most common PNS [18], which is usually associated with gynecologic, pulmonary, or lymphoma malignancies, and less frequently with esophageal or gastric malignancies [26, 32, 33]. It originates from immune-mediated responses against the cerebellum. It usually presents with a progressive onset consisting of dizziness, ataxia, dysarthria/dysphagia, diplopia and nystagmus of progressive onset with inability to walk in a few months, with cerebellar atrophy observed in imaging tests in advanced stages [34].
Although optic neuritis associated with NC is usually more related to local invasion of the orbit by the tumor in advanced stages, it has been described in patients without evidence of local invasion radiologically or histologically and its paraneoplastic origin has been postulated, but following current SPN criteria, these cases were excluded from our review [9, 35-37].
Although 5 cases in this review reported PNS appearing 1 to 5 months before the diagnosis of NPC, it is important to note that PNS can precede clinically evident malignancies by several years [30]. Therefore, thorough investigations are essential to establish an accurate diagnosis.
Treatment of PNS must be individualized. Therapy should be focused on the primary tumour. Additionally, high dose steroids and immunoglobulins may be tried in the acute phase. As it happened in most cases of this review, clinical mild improvement is common with antitumoral therapy, but long-term sequelae are frequent despite adequate tumor control. Of the 6 cases with PNS in this review whose post-treatment evolution was reflected, none had disappearance of symptoms, 3 presented partial improvement and the other presented worsening of symptoms [10, 12, 14, 15, 18].
CONCLUSIONS
PNS may present months before the diagnosis of a NC. Although rare, clinicians should consider the possibility of paraneoplastic aetiology when attending patients with NC presenting with subacute neurological symptoms once the most frequent causes had been excluded.
REFERENCES
1.Ferlito A, Elsheikh MN, Manni JJ, Rinaldo A. Paraneoplastic syndromes in patients with primary head and neck cancer. Eur Arch Otorhinolaryngol 2007; 264:211-22.
2.Zuffa M, Kubancok J, Rusnák I, Mensatoris K, Horváth A. Early paraneoplastic syndrome in medical oncology: clinicopathological analysis of 1694 patients treated over 20 years. Neoplasma 1984; 31:231-6.
3.Morales-Angulo C, Megia LR, Rubio SA, Rivera HF, Rama J. Carcinoma of the nasopharynx in Cantabria. Acta Otorrinolaringol Esp 1999; 50:381-386.
4.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol 2002; 13:1007-15.
5.Toro C, Rinaldo A, Silver C, Politi M, Ferlito A. Paraneoplastic syndromes in patients with nasopharyngeal cancer. Auris Nasus Larynx 2009; 36: 513-520.
6.Maalej M, Ladgham A, Ennouri A, Ben Attia A, Cammoun M, Ellouze R. The paraneoplastic syndrome in nasopharynx cancer: 32 cases. Presse Med 1985; 14:471-4.
7.Yang K, Zhang T, Chen J, Fan L, Yin Z, Hu Y, et al. Immune throbocytopenia as a paraneoplastic syndrome in patients with nasopharyngeal cancer. Head Neck 2012; 34:127-30.
8.Urrútia G, Bonfilll X. La declaración prisma: Un paso adelante en la mejora de las publicaciones de la revista Española de salud pública. Rev Esp Salud Publica 2013; 87(2):99-102. https://doi.org/10.4321/S1135-57272013000200001
9.Graus F, Vogrig A, Muñiz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol Neuroimmunol Neuroinflamm 2021 May 18; 8(4):e1014. https://doi.org/10.1212/NXI.0000000000001014.PMID: 34006622; PMCID: PMC8237398.
10.Phanthunchinda K, Hemachudha T, Locharoenkul C. Paraneoplastic syndrome of the nervous system. Chula Med J 1990; 34:457-465.
11.Chan KH, Leung SY, Cheung RTF, Ho SL, Mak W. Paraneoplastic motor neuropathy and inflammatory myopathy associated with nasopharyngeal carcinoma. J Neurooncol 2007; 81:93-96. https://doi.org/10.1007/s11060-006-9204-3
12.Taib BG, Kinshuck AJ, Milburn-McNulty P, Fratalia L, Forsyth L, Husband D, et al. Opsoclonus-myoclonus syndrome associated with a nasopharyngeal tumor in an adult: a case report. J Med Case Rep 2015; 9:128. https://doi.org/10.1186/s13256-015-0605-9
13.Yu ZM, Li W, Yang CQ, Song Y, Wang DY, Liu FG, Gong T. Paraneoplastic Limbic Encephalitis in a Male with Nasopharyngeal Carcinoma. Chin Med J 2016; 129:1253-4.
14.Ng SY, Kong MH, Mohamad Yunus MR. Paraneoplastic neurological disorder in nasopharyngeal carcinoma. Malays J Med Sci 2017; 24(1):113-116. https://doi.org/10.21315/mjms2017.24.1.12
15.Bhardwaj S, Khasani S, Benasher D, Stein EG, Meghal T, Jacoby N, et al. Paraneoplastic Cerebellar Degeneration in Nasopharyngeal Carcinoma: a Unique Association. Cerebellum 2019; 18:1126-1129. https://doi.org/10.1007/s12311-019-01045-1
16.Ozozen Ayas Z, Kotan D, Oguz Akarsu E, Demiryurek BE, Analysis of the Patients with Autoimmune Neurological Syndromes in a Single Center in Turkey. Osmangazi J Med 2021;43(1):62-69. https://doi.org/10.20515/otd.740327
17.Tosunoğlu B, Süleymanoğulları F, Inci ET, Çokal BG. Paraneoplastic Limbic Encephalitis with Nasopharyngeal Carcinoma: Case Report. Asian J Med Case Reps 2022; 4:9-12.
18.Stewart KE, Zeidler M, Srinivasan D, Yeo JCL Opsoclonus-myoclonus paraneoplastic syndrome in nasopharyngeal carcinoma. BMJ Case Rep 2022 Oct 26; 15(10):e250871. https://doi.org/10.1136/bcr-2022-250871.
19.Riancho J, Rodríguez-Rodríguez E, Martínez-Camerano A, Morales-Angulo C. Mielitis y anticuerpos anti-Yo: un síndrome paraneoplásico asociado al carcinoma nasofaríngeo detectado por tomografía por emisión de positrones. Descripción de un caso. Rev ORL 16(1):e32012. Disponible en: https://doi.org/10.14201/orl.32012
20.Hanağası H, Çakar A, Hanağası F, Samancı B, Tüfekçioğlu Z, Bilgiç B, et al. Emre M. Anti-Ri Associated Paraneoplastic Cervical Dystonia and Laryngospasm in a Patient with Nasopharyngeal Carcinoma. Arch Neuropsychiatry 2025; 15;62(1):94-96. https://doi.org/10.29399/npa.28517
21.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003; 49(16):1543-1554.
22.Posner J. Anti-Yo and anti-Hu. Laboratory Medicine 1999; 30(12):770.
23.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology 1992; 42:1931-1937.
24.Matschke J, Kromminga A, Erbersdobler A, Lamszus K, Anders S, Kofuncu E. Paraneoplastic cerebellar degeneration and anti-Yo antibodies in a man with prostatic adenocarcinoma. J Neurol Neurosurg Psychiatry 2007; 78:775-777.
25.Flanagan EP. Paraneoplastic Disorders of the Nervous System. Continuum (Minneap Min) 2020; 26:1602-1628. https://doi.org/10.1212/CON.0000000000000941.
26.Shams’ili S. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain 2003; 126: 1409-18.
27.Sutton IJ, Barnett MH, Watson JDG, Ell JJ, Dalmau J. Paraneoplastic brainstem encephalitis and anti-Ri antibodies. J Neurol 2002; 249:1597-8.
28.Velazquez Y, Kaur D, Ahmed A. Anti-Ri antibody paraneoplastic syndrome without opsoclonus-myoclonus. J Neurol Res 2014; 4:31-3.
29.Oh SY, Kim JS, Dieterich M. Update on opsoclonus-myoclonus syndrome in adults. J Neurol 2019, 266:1541-8. https://doi.org/10.1007/s00415-018-9138-7
30.Dalmau JO, Posner JB. Paraneoplastic syndromes. Arch Neurol 1999; 56:405-8.
31.Corsellis JA, Goldberg JG, Norton AR. Limbic encephalitis and its association with carcinoma. Brain 1968; 91:481-496.
32.Debes JD, Lagarde SM, Hulsenboom E, Sillevis Smitt PA, ten Kate FJ, Sulter GA, et al. Anti-Yo-associated paraneoplastic cerebellar degeneration in a man with adenocarcinoma of the gastroesophageal junction. Dig Surg 2007; 24:395-7. https://doi.org/10.1159/000107782
33.Meglic B, Graus F, Grad A. Anti-Yo associated paraneoplastic cerebellar degeneration in a man with gastric adenocarcinoma. J Neurol Sci 2001; 185:135-8.
34.Rojas I, Graus F, Keime-Guibert F, Reñé R, Delattre JY, Ramón JM, et al. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology 2000; 55:713-5.
35.Hoh ST, The M, Chew SJ. Paraneoplastic optic neuropathy in nasopharyngeal carcinoma--report of a case Singapore Med J 1991; 32:170-3.
36.Prasad U, Doraisamy S. Optic nerve involvement in nasopharyngeal carcinoma. Eur J Surg Oncol 1991; 17:536–540.
37.Tsai CC, Ho HC, Kau HC, Kao SC, Hsu WM. Optic neuritis: a rare manifestation of nasopharyngeal carcinoma Eye 2002;16:501–503. https://doi.org/10.1038/sj.eye.6700003